Quick Links
Minimally Invasive Mitral Valve Surgery
Sutureless Aortic Valve Replacement
Aortic Valve Repair
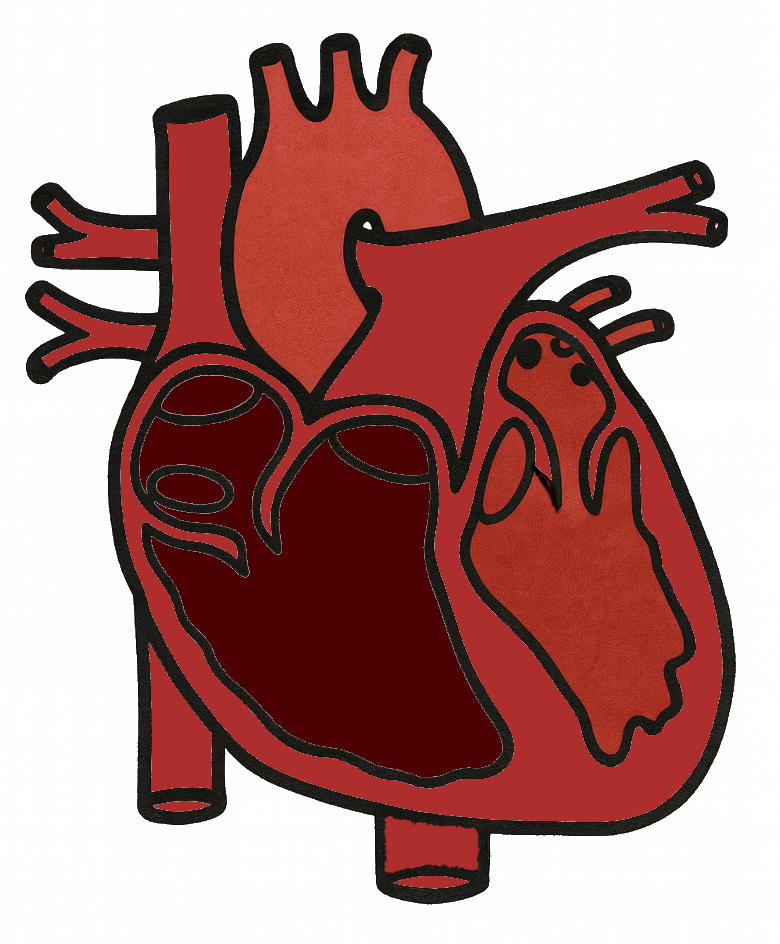
Aortic root anatomy
-
Reviews: de Kerchove et al. JTCVS 2015 & de Kerchove et al. ACS 2013
-
Aortic root (aka functions aortic annulus) – lower limit is the ventricle-aortic junction (VAJ, aka surgical annulus) & upper limit is the sinotubular junction (STJ)
-
Normal VAJ area changes during cardiac cycle – increased during isovolumetric contraction & ejection, decreased during isovolumetric relaxation, increased during diastole
-
Normal aortic annulus is oval & becomes more circular with dilation
-
The annulus is larger in a bicuspid AV than a tricuspid AV
-
The annulus becomes progressively larger with increased degree of AR

Aortic insufficiency repair classification
-
type 1 – due to dilation of the AAo ± STJ ± sinuses of Valsalva ± VAJ or cusp perforation
-
type 2 – due to leaflet prolapse (excessive cusp tissue or commissural disruption)
-
type 3 – due to leaflet restriction (BAV, calcification, fibrosis, rheumatic)

Aortic annuloplasty
-
Re-establishing normal annulus diameter is the basis for successful AV repair
-
When you need to do an annuloplasty:
-
Bicuspid AV annulus – 29-30mm
-
Tricuspid AV annulus – 28mm
-
STJ : VAJ ratio should also be between 1 – 1.5
-
Surgical dissection down to the annulus in VSARR:
-
NC sinus – limit is fibrous portion of the VAJ (aorto-mitral continuity)
-
NC/RC commissure – limit is membranous septum
-
RC sinus – limit is muscular septum (myocardium of interventricular septum & RVOT)
-
RC/LC commissure – limit is muscular septum
-
LC sinus – limit is roof of LA – the only area where dissection reaches the nadir of the leaflet hinge line
-
Options for annuloplasty:
-
Subcommissural annuloplasty (Cabrol stich)
Also increases valve coaption by closing the inter-leaflet triangles (sometimes used for this purpose instead of annuloplasty)
-
Annuloplasty is not stable over time – recurrent dilation can occur in the portions between the plication sutures – Vallabhajosyula et al. ATS 2014 & Hanke et al. JTCVS 2009
-
Also creates asymmetrical shape, abolishes normal physiology in the inter-leaflet triangles
-
External ring annuloplasty
-
teflon/Dacron ring
-
expansible external ring – Lansac et al. MMCTS 2011
-
Internal ring annuloplasty
-
Dacron strip
-
Novel preformed ring – 2yr follow-up showed good result – Mazzitelli et al. EJCTS 2015
-
Ring is close to leaflet insertion ?impingement, ?does it induce fibrosis that could extend to the leaflet

Effective & geometric height
-
standardised measurements used for cusp resuspension during VSARR
-
effective height (eH) – distance between the aorta-ventricular (basal) plane & central caption level
-
used as indicator of cusp prolapse
-
requires a normal amount of cusp tissue i.e no cusp retraction (e.g. ageing & inflammatory conditions)
-
geometric height (gH) – maximum tissue height of the cusp
-
cusp retraction defined as ≤16mm in tricuspid AVs & ≤19mm in BAVs
repair of retracted cusps do not do well & should be replaced instead

AV leaflet repair techniques
-
types of patches
-
autologous – fresh, glutaraldehyde-treated
-
xenograft – pericardium, anti-calcification, decellularised, ECM
-
synthetic – Gore-Tex, synthetic ECM
-
techniques
-
partial cusp repair – perforation, fenestration, commissure, raphe, cusp extension, unicuspid/dysmorphic valve
-
full cusp repair
-
aortic valve reconstruction (Ozaki procedure)
-
patch use is known to be associated with decreased durability of repair/recurrent AI
Aortic valve reconstruction
-
Ozaki operation is method of aortic valve reconstruction

-
Originally used glutaraldehyde-treated autologous pericardium for reconstruction
-
Cardiocel (bovine pericardium) has been used successfully in congenital heart surgery for 8 years
-
Study looked at performing the Ozaki operation using Cardiocel in sheep
-
Echo at 6 months showed valves functioning well
-
Explant showed preserved structure & stability of the Cardiocel tissue
-
Low rate of calcification
-
Neo-intima formation & re-cellularisation with host cells

Haemodynamics of David procedure
-
3D aortic root geometry & flow dynamics were assessed during the cardiac cycle in pigs with native aortic root or David procedure
-
The David root is exposed to high pressures & low shear stress for a longer time during the cardiac cycle than the native aortic root, which would favour degeneration
-
However clinical outcomes have of the David have been very good – perhaps there is leaflet remodelling that occurs in response to this pressure
-
Not presented specifically, but apparently when same model was applied to the Yacoub repair the haemodynamic profile was superior to the David & closer the native aortic root

Atrio-Ventricular Valves
Parachute ventricular partitioning
-
Used to partition off & exclude an apical aneurysm & increase EF
-
Nitinol-based PTFE covered parachute-like device
-
Transfemoral delivery
-
Guide catheter points at the landing zone in the ventricular apex, the device is deployed, & a balloon is used to expand the nitinol frame
Transcatheter Mitral Valve Implantation (TMVI)
-
Number of differences in the MV compared to the AV that makes designing transcatheter devices challenging
-
Differences in anatomy
-
Larger size & D shape
-
Complex subvavular apparatus
-
Some cords insert into the leaflet body rather than the free margin, the anterior commissure is close to the AV,
-
The MV is also close to the circumflex artery
-
Differences in physiology
-
MV separates low-resistance from high-resistance, whilst AV separates high-resistance from high-resistance
-
MV has low-resistance inflow (diastole) & high-resistance outflow (systole)
-
Annulus changes dimensions up to 40% in systole
-
Both aetiology & affected structures are highly varied
-
Differences in design priorities
-
Aim to decrease or maintain the EOA, as opposed to maximising
-
The height of the device affects risk of LVOTO
-
The angle of the device to the AV also affects risk of LVOTO
-
Size of ventricle affects how much room there is for the device
TMVI – CardiAQ (Edwards Lifesciences)
-
Bovine pericardial trileaflet valve with intra-annular sealing skirt to minimise PVL
-
Mostly sits in the LA to avoid risk of LVOTO
-
Transeptal & transapical delivery
-
Steps: Leaflet capture, vale delivery, valve expansion
-
Used in 8 patients so far with good success rate, reduction of MR to trace/none, low gradient, trace/no PVL
-
Currently undergoing FDA early feasibility study & CE mark study
-
http://circinterventions.ahajournals.org/content/8/7/e002135.extract
TMVI – FORTIS Valve (Edwards Lifesciences)
-
Bovine pericardial trileaflet valve, D shaped frame with circular valve
-
Transapical delivery
-
Steps: paddles partially deployed in ventricles, leaflets captured, flange release, valve released
-
FDA early feasibility study initially stopped due to valve thrombus, though anticoagulant regimen revised & study restarted
TMVI – Tiara Valve (Neovasc Inc)
-
D shaped valve, anterior portion faces the aortic valve
-
Steps: atrial skirt deployed first, device is centred & orientated, then ventricular skirt deployed
-
Used in 7 patients in Canada in special access scheme with good success
-
Currently undergoing the TIARA-I early feasibility study
TMVI – Tendyne Valve (Tendyne)
-
D shaped outer stent with circular inner stent, apically teathered
-
Transapical delivery, performed mostly with live 3D echo
-
Steps: valve deployed 80% in the atrium, device rotated to orientate correctly, withdrawn into the annulus, teather secured to the apical apex
-
Optimal tension on the teather is still unclear, but based on PVL & strain
-
17 implants so far
-
http://interventions.onlinejacc.org/article.aspx?articleid=1905024
TMVI – HighLife Procedure
-
2 step procedure:
-
Sub-annular ring implantation
-
Valve-in-ring implantation
-
All chordae should be caught in the ring
-
Ventricular part delivered first, then whole valve is pushed up into the annulus, then atrial part delivered
-
Transapical or transatrial delivery
-
Self centuring & self positioning on release
-
First in man planned for next month
Tricuspid valve repair
-
The TV tends to dilate in the anterior & posterior leaflet direction (I.e not in the septal leaflet direction)
-
Bicuspidation of the tricuspid valve technique
-
Mattress sutures from the mid point of the posterior leaflet to the mid point of the septal leaflet
-
No difference in survival compared to annuloplasty
-
Mitralign transcather device for tricuspid annuloplasty
-
Insertion made using RF
-
First insertion make around the posterior-septal commisure
-
Device is sinched down 2 plegetted sutures & creates annuloplasty
-
Max distance to sinch (I.e between sutures) is 2.8cm – the tricuspid annular tissue is more fragile than mitral annular tissue, & any more distance could be damaging
-
Makes the posterior leaflet redundant
-
Used in 10 patients worldwide
-
Tricuspid valve repair using extra cellular matrix cylinder
Heart Failure Surgery
Heartmate III CE mark trial
-
Uses Maglev technology to magnetically suspend the impeller (no hydrodynamic or mechanical bearings) – allows for wide range of flow, artificial pulse (hopefully less aortic insufficiency, blood stasis, fewer GI bleeds), more consistent pump gaps (hoping to reduce haemolysis & thrombosis)
-
First in-human trial, prospective, non-randomised, n=60
-
Mean age 59yrs, ischaemic aetiology in 44%
-
All implants via median sternotomy
-
Drive line externalised with silicone to skin interface in 96% (designed to reduce infection)
-
42% had concomitant procedures (valve operations, PFO, LAA occlusion)
-
mean CPB time 84min (63-110)
-
30 day outcomes
-
Bleeding 30%
Quite high due to strict definition (≥4 units in first 7 days, ≥1 unit after day 7)
-
12% required reoperation
-
Stroke 4% – 1 patient had difficulty engaging inflow conduit; 1 ischaemic stroke from anaphylactic shock
-
8% right heart failure (2 requiring RVAD support)
-
No device malfunction, thrombosis, haemolysis
-
98% survival (1 death in the ischaemic stroke patient)
-
6 month endpoint also met, awaiting CE mark approval
-
In Viena (where study was) as soon as CE mark they will stop implant Heartmate II & only use III
Lavare cycle in the HeartWare HVAD
-
Lavare cycle involves periodic speed modulation which may reduce blood stasis
-
Lower speed (-200rpm) for 2 sec, then higher speed (+200rpm) for 1 sec, then baseline for 60sec, then cycle starts again
-
Step 1 – large vortex results in ventricular washout
-
Step 2 –
-
Step 3 – large ventricular washout again then normalised flow
-
ReVOLVE registry (n=248) analysis of the Lavare cycle
-
Significantly fewer stroke, sepsis, RHF
-
Other outcomes including survival were similar
-
Usually turned on after the patient leaves the OR (in Vienna)
Novel inflow cannula implant technique
-
LVAD results in servely disrupted blood flow in the LV – bloods flows to the ventriclar apex instead of the AV valve, increasing the risk of thrombosis
-
Study used an ex vivo dilated porcine heart (non-beating) on ECMO 4.5L
-
A cone shaped prosthetic tube was attached to the mitral valve annulus to funnel blood directly into the LVAD & avoid the disturbed flow in the LV
-
Resulted in higher flow rate & more streamlined elliptical shaped flow
-
Acute live animal experiment – successful implanted & weaned off CPB (onto VAD only) (animal had to be terminated at 1 hr due to ethics)
-
Next step is chronic animal model
LVAD less invasive approaches
-
Upper hemi-sternotomy + left thoracotomy approach possible
-
Helps preserve sternum for later heart transplantation
-
Alternative to hemi-sternotomy is right thoracotomy or right parasternal incision
-
Disadvantage is the outflow graft has to cross the midline twice as it travels to aorta
-
Disadvantage is more difficult access
-
Advantage is sternum is even further preserved for heart transplantation
-
Related paper: Maltais et al. ACS 2014
Minimally Invasive Mitral Valve Surgery
Debate for sternotomy approach – David Adams, Mount Siani Medical Center, NY
-
Achieving 100% repair rate with no residual regurgitation is the most important priority
-
Some studies of the mini-thoracotomy approach have compromised repair rate or an increased rate of residual MR (3 or 4+) post repair
-
Handling complexities such as annular calcification is much more difficult through a mini-thoracotomy
-
To achieve good outcomes with mini or robotic mitral surgery you need to be at a super-high volume centre, which most places are not
-
Meta-analyses have found increased rate of stroke, possibly due to retrograde perfusion from femoral CPB cannulation
-
Limited sternotomy is not as morbid as the traditional full sternotomy but provides the same full open access, & has smaller incision with good cosmesis
Debate for minimally invasive approach – Patrick Perier, Herz und Gefäß Klinik, Germany
-
Mini-thoracotomy with direct vision still requires rib spreading which is still morbid & not as ‘minimally invasive’ as possible – so it should be done as port-access surgery with video vision
-
The exact same repair techniques should be replicated with the mini-thoracotomy approach
-
New programs need to be highly selective of simple patients at the beginning, then progressively add more complex repairs & concomitant procedures (e.g. AF) as they gain experience
Evidence on mini-mitrals – J Grau, Cleveland Clinic, USA
-
Paper: Bolling et al. ATS 2010
-
Minimum of 40 mitral operations per surgeon per year are needed to achieve an 80% repair rate (deemed minimum acceptable rate)
-
Learning curve for minimally invasive approach at very high volume centre (Leipzig) was 150-200 patients
-
Learning curves are surgeon-specific as well, & some required re-mentoring
-
Minimum of 50-100 mini-mitrals per year are needed to be proficient
-
Very difficulty achieving this number as you need to be an expert in MV repair first, then expert in minimally invasive approach
Robotic-assisted mitral valve surgery
-
Robotic assistance provides many advantages in visualisation, control and precision allowing for minimally invasive repair of the most complex valve
-
Video by Didier Loulmet demonstrated repair techniques
-
They use a fluorescence covered endoaortic balloon (for CPB) to facilitate positioning
-
Paper: Yaffee et al. JTCVS 2015
-
Related systematic review: Seco et al. ATS 2013
Sutureless Aortic Valve Replacement
Intuity valve (Edwards)
-
MIS AVR meta-analyses:
-
lower mortality, fewer transfusion, shorter HLOS
-
longer XC & CPB times
-
Intuity valve – essentially a Magna Ease valve (Edwards) combined with stainless steal stent & sealing cloth from the Sapien 3 valve
-
CADENCE MIS trial http://www.ncbi.nlm.nih.gov/pubmed/25441065
-
Intuity valve via upper hemi-sternotomy (n=51) vs. standard AVR via full sternotomy (n=49)
-
3 conversions to full sternotomy with scented bioprostheses – 2 unable to fit valve properly, 1 annulus tear
-
24% relative reduction in XC time (despite mini-incision, which usually increases XC time by 16%)
-
mortality not significantly different
-
PPM rate not significantly different, despite stent extending lower into the ventricle. Percival valve (similar sutureless valve) has higher PPM rate, but study had higher mean age than CADENCE-MIS (78 vs. 73), which may account for difference?
-
increased rate of mild PVL – potentially due to improper sizing, not aggressively debriding annular calcification
-
Intuity had significantly better EOA & gradients
Management of small aortic annulus
-
retrospective study comparing stented valves, Manougian procedure, stentless Freestyle prosthesis, & sutureless Percival S valve in patients with annulus <21mm
-
stentless group had significantly lower mean aortic gradient & higher EOA than other groups
this was a full root replacement with Freestyle graft, allowing for a larger prosthesis than the annulus
- the Trifecta valve (St Jude) had significantly lower gradients & higher EOA than perimount, magna ease & mitroflow prostheses
Timing of individual steps in SAVR
-
aim to identify specific steps where time could be saved
-
exposure (stating after XC + AV assessment) – 5.3mm (10min)
-
resection (resection of AV leaflets + annulus decalcification + sizing) – 8.1mm (16%)
-
suturing – 17.3mm (33%)
-
tying – 9.1min (18%)
-
declamping – 11.9min (23%)
-
sutureless valves & knot tying devices have potential to significantly reduce time
-
more experienced surgeons were faster